Tuesday, February 09, 2010
Wednesday, February 01, 2006
Lecture Summary: February 1
We briefly reviewed formal charge, then examined how oxidation numbers as an alternative scheme for deciding how the electrons (charge) is distributed around a molecule. Both schemes are very useful to chemists as predictive tools, but they are really just simple stories. To know more, you would have to do quantum mechanical calcualtions to determine where the electrons are located. These sorts of things can be done, but you don't want to have to do them for every molecule you are interested in. The myths have a use!
Tuesday, January 31, 2006
Weird Words of Science: The Ties that Bind
Many transition metals react with bases (such as ammonia) to produce beautifully colored transition metal-ligand complexes. The word ligand comes from the Latin ligare which means to tie or bind. The same root leads to ligaments, which tie your bones together.
Weird Words of Science: Tooth and Claw - Chelation
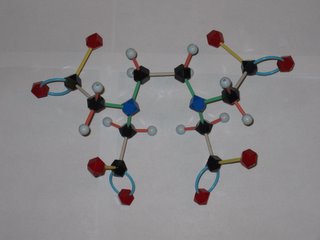
There has been a lot news coverage lately about the abuse of chelation therapy for treating autism and other disorders. So what's a chelate and how does it work to remove metal ions from the body? EDTA is shorthand for ethylenediamminetetraacetic acid, which has the structure shown at the left. The disodium calcium salt of EDTA is the usual chemotheraputic form. Lone pairs of electrons on the nitrogens and oxygens of the EDTA (tagged blue and red in the photo) latch onto the metal. This Lewis acid-base reaction results in the metal being sequestered inside the EDTA molecule. Tucked away inside the EDTA, the metal can't accumulate in the body's tissues and is eventually eliminated. EDTA has different affinities for different metal ions, but is a pretty effective scavenger of most metal ions, including iron and calcium. Later this semester we will learn more about how to tell which metals will be removed more efficiently. Removal of too much calcium can result in cardiac arrest, so EDTA is not without safety isses!
The word chelation come from the Greek for claw. Molecules that attach to metals at multiple points, like EDTA, are called multidentate ligands from their capacity to "bite" onto the metal. EDTA makes a hexadentate metal-ligand complex (6 points of attachment) with some ions, a pentadentate complex with others.
The photo is courtesy of a Chem 104 student!
Monday, January 30, 2006
How to look like a chemist: Drawing chemical structures
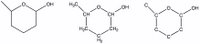
Chemists often don't show all the hydrogen atoms in a molecule, since they can clutter up the picture (and often aren't involved in the reaction). Often they use representations that omit the carbons, assuming that carbons are at the end of any line segment (see the structure on the far left). If they want to indicate hydrogens, often they will embed the H's with the carbons, as in the middle structure. The structure at the far right is unlikely to be drawn by a practicing chemist - who would use either of the two models on the left!
How to talk like a chemist: Hydroxide, hydroxyl? Aren't they the same thing?
 The glucose molecule shown at left has lots of OH's on it. Are these hydroxides? Technically, no. Hydroxides are ions, individual OH- units, either in solution or in a crystal. In glucose, the OHs are termed hydroxyl groups, where the OH is bound covalently to the molecule. The name gives a clue to the chemical structure and expected reactivity!
The glucose molecule shown at left has lots of OH's on it. Are these hydroxides? Technically, no. Hydroxides are ions, individual OH- units, either in solution or in a crystal. In glucose, the OHs are termed hydroxyl groups, where the OH is bound covalently to the molecule. The name gives a clue to the chemical structure and expected reactivity!Alchohols have as a distinguishing feature a hydroxyl group, and therefore don't behave as bases (losing hydroxide ions) like sodium hydroxide would, even though their chemical formulas might look similar. For example, compare the formulas for sodium hydroxide (NaOH) and ethyl alchohol (CH3CH2OH).
Lecture Summary 1/29: Formal Charges & Redox
We discussed how formal charges are a useful predictive tool, particularly for organic compounds. The charges are not real, in the sense that they cannot be experimentally determined, but the formalism is very useful in organizing and understanding organic reactions. We began to consider oxidation-reduction (redox) reactions, noting that Ag+ ions react with metallic copper to produce metallic silver and Cu2+ ions. Breakfast cereal manufacturers take advantage of the redox reaction of metallic iron with acid to produce bioavailable Fe2+ ions!
Friday, January 27, 2006
Example Problem 2: Nucleophiles/Electrophiles and Penicllin

Penicillin was one of the first antibiotics in wide use. It was discovered in the late 19th century by a French medical student (Ernest Duchesne), though his work was never pursued. Fleming independently discovered the antibacterial activity of Penicillium mold derivatives in 1928. The active molecule was difficult to extract. The compound was finally synthesized in 1957 by John Sheehan, a chemist at MIT. This feat was made possible by the determination of penicillin's structure in 1944 by Dorothy Crowfoot Hodgkin, an X-ray crystallographer who won the 1964 Nobel prize in chemistry for that discovery and many others (including B-12 and insulin).
How does penicillin work? It is a Trojan horse molecule. Penicillin disrupts the synthesis of bacterial cell walls, thus inhibiting the bacteria's reproduction. The enzyme responsible for assembling the cell walls picks up penicillin, thinking it can incorporate into the wall. Unfortunately for the bacteria, the penicillin molecule opens up and destroys the enzyme's ability to function.
The key step in this sneak attack is the nucleophilic attack of the enzyme onto an electrophilic site on the penicillin. Identify the possible electrophilic sites on penicillin. Which site is most likely to react and why? Draw the structure that results from the attack of an ROH (where R is a big group - the whole enzyme) molecule onto the critical site on penicillin.
Watch this 15 minute webcast to see the solution.
Lecture Summary 1/27: The ties that bind - metal/ligand interactions

Our understanding of inorganic reactions also benefits from considering Lewis acid/base interactions. The ligand in a metal complex is acting as a Lewis base, donating an electron pair to the metal, which is typically positively charged. Many of these complexes are highly colored. Cisplatin, a platinum complex of ammonia and chloride is used to treat many cancers.
Lecture Summary 1/25: The 'basics' of organic chemistry - nucleophiles and electrophiles
Many organic chemistry reactions can be understood in terms of nucleophiles (molecules with lone pairs in search of partial positive charges) and electrophiles (molecules with sites that have partial positive charges that would welcome an electron pair). We looked at the synthesis of oil of wintergreen, a fragrant compound that is formed when a carboxylic acid reacts with an alcohol to produce an ester. We saw examples of using arrow to "push" electrons around to understand at a more fundamental level what is happening.
