Example Problem 2: Nucleophiles/Electrophiles and Penicllin
Penicillin was one of the first antibiotics in wide use. It was discovered in the late 19th century by a French medical student (Ernest Duchesne), though his work was never pursued. Fleming independently discovered the antibacterial activity of Penicillium mold derivatives in 1928. The active molecule was difficult to extract. The compound was finally synthesized in 1957 by John Sheehan, a chemist at MIT. This feat was made possible by the determination of penicillin's structure in 1944 by Dorothy Crowfoot Hodgkin, an X-ray crystallographer who won the 1964 Nobel prize in chemistry for that discovery and many others (including B-12 and insulin).
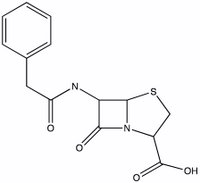
How does penicillin work? It is a Trojan horse molecule. Penicillin disrupts the synthesis of bacterial cell walls, thus inhibiting the bacteria's reproduction. The enzyme responsible for assembling the cell walls picks up penicillin, thinking it can incorporate into the wall. Unfortunately for the bacteria, the penicillin molecule opens up and destroys the enzyme's ability to function.
The key step in this sneak attack is the nucleophilic attack of the enzyme onto an electrophilic site on the penicillin. Identify the possible electrophilic sites on penicillin. Which site is most likely to react and why? Draw the structure that results from the attack of an ROH (where R is a big group - the whole enzyme) molecule onto the critical site on penicillin.
Watch this 15 minute webcast to see the solution.


0 Comments:
Post a Comment
<< Home